1
Divisions of chemistry
The five main branches of chemistry
1. Organic chemistry
2. Inorganic chemistry
3. Analytical chemistry
4. Physical chemistry
5. Biochemistry
2
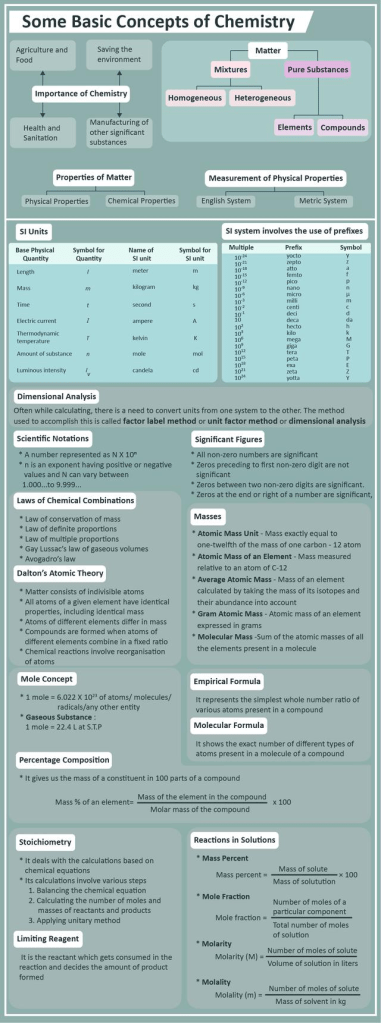
Laws of chemical combination
- Law of conservation of mass: this law states that matter can neither be created nor destroyed. In other words, the total mass, that is, the sum of mass of reacting mixture and the products formed remains constant.
- Law of definite proportion: This law states that if two elements combine to form more than one compound, the masses of these elements in the reaction are in the ratio of small whole numbers.
- Gay lussac’s law of gasseous volume: This law states that when gases are produced or combine in a chemical reaction, they do so in simple ratio by volume given that all the gases are at same temperature and pressure
- Avogadro’s law: It stated that under same conditions of temperature and pressure, equal volume of all the gases contain equal number of molecules.
- Law of multiple proportions: When two elements combine with each other to form two or more compounds, the ratios of the masses of one element that combines with the fixed ratio of the other are simple whole numbers.
3
Dalton’s atomic theory
According to Daltons atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms. The postulates of this theory may be stated as follows:
(i) All matter is made of very tiny particles called atoms.
(ii) Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
(iii) Atoms of a given element are identical in mass and chemical properties.
(iv) Atoms of different elements have different masses and chemical properties.
(v) Atoms combine in the ratio of small whole numbers to form compounds.
(vi) The relative number and kinds of atoms are constant in a given compound.
Limitations of Dalton’s atomic theory :
- Atoms of the same or different types have a strong tendency to combine together to form a new group of atoms. For example, hydrogen, nitrogen, oxygen gases exist in nature as group of two atoms. This indicates that the smallest unit capable of independent existence is not an atom, but a group of atoms.
- With the discovery of sub-atomic particles, e.g.,electrons, neutrons and protons, the atom can no longer be considered indivisible.
4
Significant figures
Number of digits in a figure that express the precision of a measurement instead of its magnitude. The easiest method to determine significant digits is done by first determining whether or not a number has a decimal point.
5
Stoichiometric coefficients
In a balanced reaction, both sides of the equation have the same number of elements. The stoichiometric coefficient is the number written in front of atoms, ion and molecules in a chemical reaction to balance the number of each element on both the reactant and product sides of the equation.
CH4+2O2→CO2+2H2O
6
Mole
A mole of a substance is defined as the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12 g of C12.
Mole in terms of volume:
One mole of all gaseous substances at 273 K and 1 atm pressure occupies a volume equal to 22.4 liter or 22,400 ml. The unit of molar volume is liter per mole or milliliter per mole.
Avogadro Number :
The number of particles present in 1 mole of any substance is fixed with a value of 6.023×10^23 This is known as Avogadro number or constant represented by No.
7
Limiting reagent
The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. From the reaction stoichiometry, the exact amount of reactant needed to react with another element can be calculated. If the reactants are not mixed in the correct stoichiometric proportions (as indicated by the balanced chemical equation), then one of the reactants will be entirely consumed while another will be left over. The limiting reagent is the one that is totally consumed; it limits the reaction from continuing because there is none left to react with the in-excess reactant.